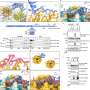
Recent research has unveiled that when ribosomes, the cell’s protein manufacturing units, encounter disruptions, cells activate emergency stress defenses. This finding sheds light on the intricate mechanisms that govern protein synthesis and cellular responses to stress.
Ribosomes play a crucial role in translating genetic information from messenger RNA (mRNA) into proteins. They operate by binding to mRNA and reading its sequence, linking amino acids to assemble proteins essential for life. However, when ribosomes collide or encounter obstacles during this process, it can lead to significant cellular stress.
Understanding Ribosomal Stress Responses
According to a study conducted by the Research Institute of Molecular Genetics, published in September 2023, cells have evolved sophisticated strategies to cope with the malfunction of ribosomes. When these protein factories face challenges, such as collisions with other ribosomes or disruptions in the mRNA strand, the cell initiates a protective stress response. This response is vital for maintaining cellular function and preventing potential damage.
In the study, researchers observed that ribosomal stalling triggers the activation of specific signaling pathways. These pathways lead to the expression of genes responsible for stress resistance. The ability to adapt to ribosomal stress is particularly important in rapidly dividing cells, which require efficient protein synthesis to support growth and development.
The researchers employed advanced imaging techniques to visualize ribosome collisions in live cells. Their observations indicated that ribosomal encounters can lead to significant disruptions in protein production, prompting the cell to respond swiftly to restore normal function.
The Broader Implications for Cellular Biology
The ramifications of these findings extend beyond mere cellular mechanics. Understanding how ribosomes respond to stress can provide insights into various health conditions, including cancer and neurodegenerative diseases. Disruptions in protein synthesis are often implicated in these diseases, making it crucial to comprehend the underlying processes.
Moreover, the research emphasizes the importance of ribosome integrity in cellular health. When ribosomal function is compromised, it can lead to a cascade of cellular dysfunction. The ability of cells to launch a stress response may represent a potential therapeutic target for diseases where protein synthesis is disrupted.
As scientists continue to explore the complexities of ribosomal function and stress responses, this study marks a significant step forward in cellular biology. It highlights the resilience of cells in the face of adversity and underscores the intricate relationship between ribosomes and cellular health.
The findings could pave the way for future research aimed at developing interventions that enhance cellular stress responses, potentially leading to novel treatments for diseases associated with impaired protein synthesis.