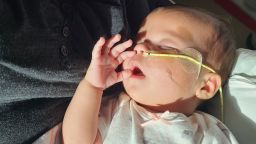
A troubling situation has surfaced regarding the sale of recalled baby formula, specifically the ByHeart Whole Nutrition powdered infant formula, which was pulled from shelves due to serious health concerns. Despite a nationwide recall issued on November 11, 2023, instances of the recalled product were still found on store shelves as late as December, raising significant safety concerns for parents.
During a routine grocery trip, a concerned shopper spotted a can of ByHeart formula at a local Kroger store, with a recall notice from November visibly taped underneath. This prompted immediate reactions from food safety experts, including attorney Bill Marler, who is representing families affected by infant botulism linked to this formula. Marler was in the process of amending complaints against retailers, stating they failed to act swiftly to remove the hazardous product.
Kroger responded to the discovery, asserting that they had taken steps to remove the product and implemented measures to prevent customers from purchasing it. The company stated, “When the recall was issued, we urgently removed the affected product and immediately placed a block at the point of sale to make it impossible for a customer to purchase the recalled item.” The statement did not clarify why the formula remained on the shelf despite these protocols.
The issue extends beyond Kroger, as the U.S. Food and Drug Administration (FDA) issued warning letters to several retailers, including Target and Walmart, on December 12, 2023. Inspections revealed that cans of ByHeart formula were still being sold in stores across 36 states after the recall was announced.
Problems with recalled products lingering on shelves are not uncommon. In 2022, the Consumer Product Safety Commission (CPSC) fined TJX, the parent company of TJ Maxx and Marshalls, $13 million for selling over 1,200 recalled items, including dangerous infant sleepers. A spokesperson for TJX emphasized their commitment to product safety, noting improvements in their recall processes.
CPSC Acting Chairman Peter Feldman indicated that lingering recalled products present a significant challenge. “When CPSC recalls a product, it becomes illegal to sell that product,” he stated. Compliance issues often arise, either through inadequate communication with staff or lapses in inventory management. In some cases, retailers have been accused of turning off inventory control systems during busy sales periods, which can lead to the sale of recalled goods.
To combat this issue, the CPSC and the FDA conduct recall effectiveness checks, although these are not consistently performed. The CPSC’s dedicated ESAFE team monitors online platforms for the sale of recalled products, issuing approximately 33,000 takedown orders in just three months—representing a 150% increase from the previous year.
Particular attention is warranted for categories like baby products and electronics, which often remain in circulation long after a recall. Feldman urged parents to verify the safety of secondhand items by consulting the CPSC website. He noted that in fiscal year 2025, the CPSC recorded 357 recalls, a significant increase from 238 recalls in 2020.
The FDA’s response to the ByHeart situation has been scrutinized. Following an investigation into a surge of infant botulism cases, the agency was slow to broaden the recall initially. State and federal regulators suspected that the limited scope of the initial recall contributed to confusion among retailers regarding the necessity to remove all lots from shelves.
Infant botulism is a severe condition that occurs when infants ingest spores of the botulinum bacteria, which can lead to paralysis. Symptoms can take weeks to manifest and may include constipation, fussiness, and muscle control loss. The FDA has reported that as of December 17, 2023, there were 51 confirmed cases across 19 states, resulting in hospitalizations, although no fatalities have been reported.
While a treatment known as Baby Botulism Immune Globulin, or BabyBIG, has been effective in combating the toxin, its high cost and the extensive hospital stays required for recovery raise further concerns for affected families.
In response to the ongoing crisis, ByHeart released a statement apologizing for the distress caused and confirmed that they have paused production while investigating the contamination source. They urged parents to monitor for symptoms of infant botulism diligently.
As the situation evolves, advocates like Sandra Eskin, CEO of the nonprofit advocacy group Stop Foodborne Illness, emphasize the need for stronger accountability. “A recall is the last line of defense, separating consumers from foodborne illness. But if companies can’t get it right, we are really in trouble,” she remarked, underscoring the urgent need for improved safety measures in the food industry.
The ongoing challenges surrounding product recalls illustrate the critical importance of vigilance among both consumers and retailers in ensuring that dangerous products do not remain available to the public.