The gene p53, known as the guardian of the genome, plays a critical role in preventing cancer by maintaining genomic stability. However, mutations in this gene can transform its function from protector to promoter of cancer. A recent study conducted by researchers at Baylor College of Medicine has unveiled that certain p53 mutations may increase tumor vulnerability to immunotherapy, potentially guiding more effective treatment strategies.
Published in Communications Biology, the study focused on two prevalent p53 mutations, R273H and R175H, and their impact on cancer cell growth. Dr. Weei-Chin Lin, a professor of molecular and cellular biology and medicine at Baylor’s Dan L Duncan Comprehensive Cancer Center, led the research. The team investigated how these mutations influenced DNA replication, a crucial step in cell proliferation.
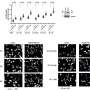
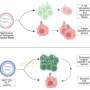
The findings revealed that the R273H mutation promotes excessive DNA replication, leading to aggressive cancer growth. Interestingly, this process simultaneously triggers a robust immune response against the cancer cells, activated through the cGAS-STING pathway. In contrast, the R175H mutation facilitated cancer growth without initiating any immune response.
Understanding these differences is vital as they can significantly affect treatment outcomes. “This highlights the importance of understanding the specific type of p53 mutation in each patient’s tumor,” Lin noted. His team hypothesized that tumors with the R273H mutation might respond better to immune checkpoint inhibitors, therapies that help the immune system combat cancer.
To test this, researchers employed mouse models of breast cancer. The results were promising: tumors with the R273H mutation treated with immune checkpoint inhibitors exhibited an increase in CD8 + T cells, which are crucial for fighting cancer, and showed signs of active cancer cell destruction.
These insights may pave the way for personalized cancer therapies. Although immune checkpoint inhibitors have transformed treatment for various cancers, they do not benefit all patients. Lin emphasized, “Our study suggests that combining immunotherapy with drugs targeting DNA replication could further enhance the immune response.”
While further research is necessary before these findings can be translated into clinical practice, they offer a glimpse into the future of personalized cancer treatment. Identifying tumors with specific mutant p53 variants may enable physicians to predict which patients are likely to respond favorably to immunotherapy, enhancing the effectiveness of cancer treatments.
The research team, including first author Kang Liu and collaborators Lidija A. Wilhelms Garan and Fang-Tsyr Lin, contributed significantly to this study, which underscores the importance of precision medicine in oncology. With ongoing advancements, the hope is to provide tailored therapies that improve patient outcomes and survival rates in the fight against cancer.