A team of researchers at the University of Minnesota has developed an innovative one-pot method to synthesize blue light-responsive aryne precursors from carboxylic acids. This breakthrough could significantly simplify the process of designing complex aromatic compounds, which are crucial in fields such as drug discovery and agricultural chemistry.
Arynes, known for their unique structural features, are highly reactive organic intermediates characterized by a triple bond within an aromatic ring. Their ability to form bonds with various functional groups makes them valuable in synthetic chemistry. Despite this potential, synthetic chemists have often avoided using arynes due to the complexities involved in their synthesis.
Challenges in Aryne Synthesis
Traditionally, creating arynes has required harsh conditions, including strong bases that strip protons from C–H bonds, followed by halide elimination. This multi-step process is unsuitable for molecules with sensitive functional groups and poses safety risks. Additionally, previous methods involving thermally activated precursors have proven to be explosive, while ultraviolet-light methods often resulted in unwanted side reactions.
The research team, led by Chris M. Seong, aimed to fill the gap left by these limitations. They explored the potential of using o-iodoniobenzoates as a starting point for aryne precursors, focusing on achieving a method that was both simple and safe.
Innovative One-Pot Synthesis
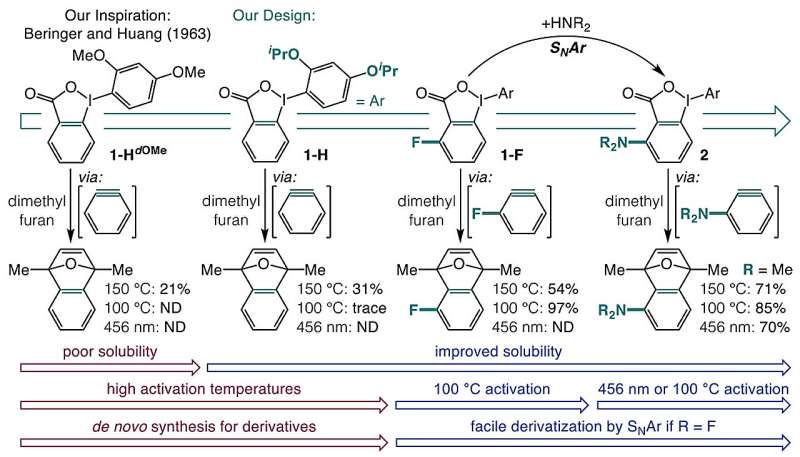
The researchers devised a one-pot synthesis that transforms carboxylic acids into o-iodoniobenzoate precursors, which can then be activated using visible light or mild heat. However, they initially faced challenges due to the poor solubility of o-iodoniobenzoates and their tendency to undergo side reactions.
Through experimentation, the team discovered that introducing isopropoxy groups enhanced solubility and minimized unwanted reactions. Furthermore, adding a substituent adjacent to the carboxylate group allowed for the successful activation of aryne precursors through blue light or heating to 100 °C.
The activation mechanisms differ depending on the method used. Heat activation primarily relies on a field effect, where nearby chemical groups create an electronic field that facilitates decarboxylation. In contrast, blue light at a wavelength of 398 nm excites the molecule to a triplet state, prompting the aromatic ring to detach from iodine, resulting in the formation of arynes.
This new approach not only unlocks access to a wide array of aryne precursors starting from readily available carboxylic acids but also demonstrates compatibility with various functional groups. The implications for simplifying the synthesis of complex aromatic compounds are significant, potentially transforming research in pharmaceuticals and agrochemicals.
The findings, published in the journal Nature, represent a significant advancement in synthetic chemistry. The research team emphasizes that their one-pot synthesis method opens the door to previously unexplored chemical spaces, enabling the development of new compounds that could lead to innovative applications in various industries.
This work was reviewed and fact-checked to ensure accuracy and credibility, reflecting the rigorous standards of scientific reporting. Further details are available in the publication by Chris M. Seong et al., titled “Myriad Aryne Derivatives from Carboxylic Acids” in Nature (2025).