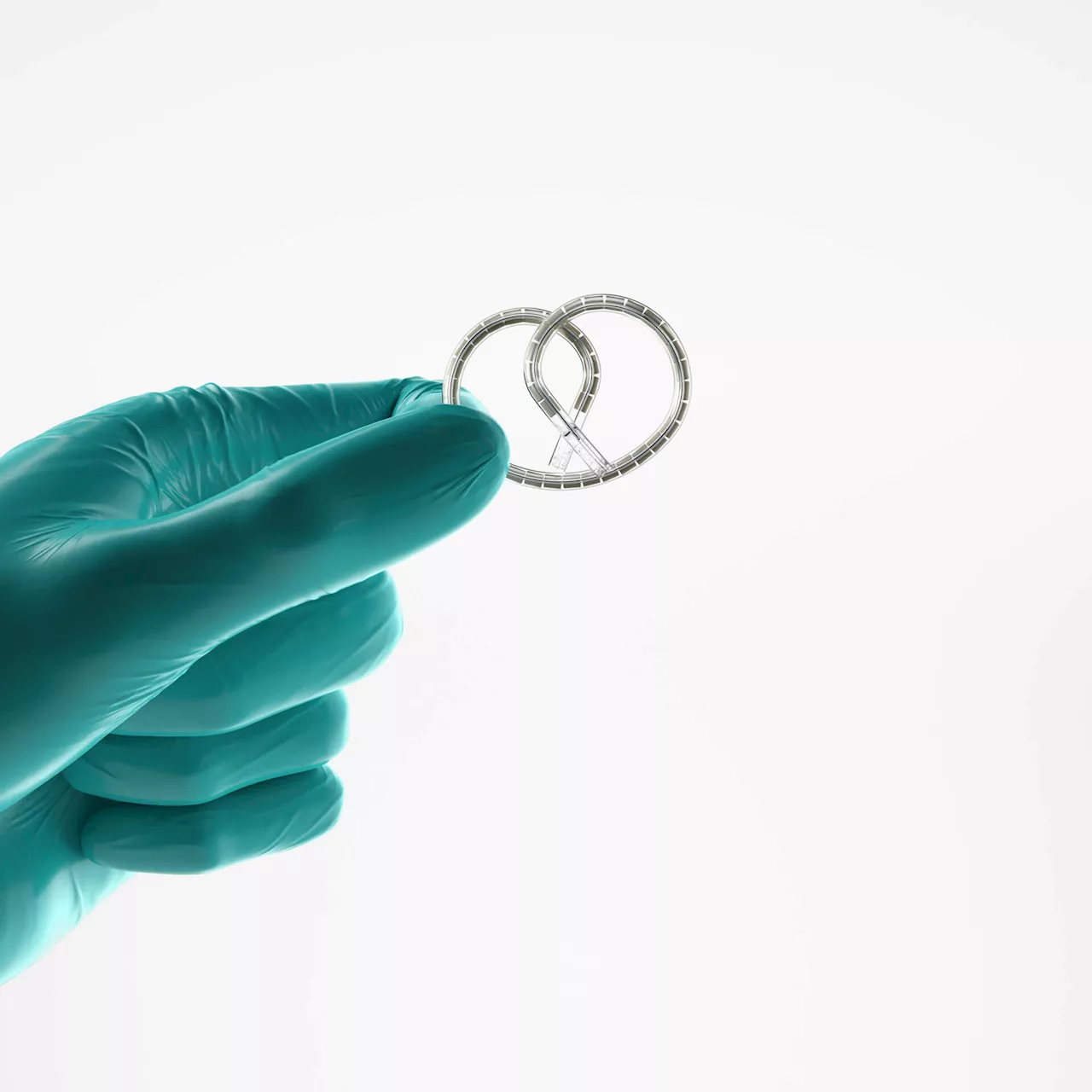
The U.S. Food and Drug Administration (FDA) has approved a groundbreaking implant designed to aid patients with bladder cancer, marking a significant advancement in treatment options. The device, known as Inlexzo, is shaped like a pretzel and slowly dispenses cancer-fighting medications directly within the bladder. This approval, announced on September 9, 2025, is particularly noteworthy for patients suffering from the most common form of bladder cancer, as it represents the first major development in over five decades.
For many years, patients whose bladder cancer did not respond to chemotherapy or other treatments faced a grim choice: undergo a cystectomy, a surgery that removes part or all of the bladder. This operation often leads to life-altering consequences, including reliance on an external urinary pouch. Dr. Chris Cutie, a former urologist at Mass General Brigham and a key figure in the device’s development, described the procedure as “very onerous.”
Dr. Cutie highlighted the challenges faced by scientists attempting to create effective treatments for bladder cancer. “The bladder is really good at evacuating things. It doesn’t like to keep things in,” he explained. Traditional approaches to delivering medication involved injecting drugs directly into the bladder and then requiring patients to delay urination to maximize treatment exposure. This method was not only uncomfortable but also largely ineffective.
The introduction of Inlexzo offers a new approach. In a small clinical trial, 82% of patients who received the implant in combination with a drug experienced complete remission of their cancer, with more than half remaining cancer-free after at least one year. This promising data comes from Johnson & Johnson, the company collaborating with Dr. Cutie and the TAURUS group to refine the device.
Dr. Cutie, now a vice president at Johnson & Johnson, expressed pride in the device’s capabilities. “What we’re really proud of is that we see complete responses—meaning the disease is completely eradicated—within the first three months of treatment,” he said. This rapid response is particularly significant for patients like Bill Parisi, who was diagnosed with bladder cancer in 2021 and initially faced the prospect of a cystectomy.
Parisi, concerned about the impact of such surgery on his ability to run his business, opted to participate in the clinical trial after exhausting typical treatment options. He received the Inlexzo implant and underwent refilling procedures every three months. While he experienced some irritation from the device, the ability to retain his bladder made the discomfort worthwhile. After 18 months of treatment, Parisi was declared cancer-free, and the implant was subsequently removed.
Despite the encouraging trial results, experts urge caution. Dr. Adam Kibel, chair of urology at Mass General Brigham, acknowledged the potential benefits of Inlexzo but emphasized the need for further research to assess its long-term impact on quality of life and survival rates. “This does a better job of lengthening exposure to cancer-fighting drugs,” he noted, but questioned whether patients would adapt to having a device in their bladder.
As research continues in the field of bladder cancer treatment, the introduction of Inlexzo represents a potential turning point for patients who have historically faced limited options. The hope is that this innovative device could change the landscape of bladder cancer care, providing patients with a more effective alternative to invasive surgery while addressing the challenges of drug delivery.