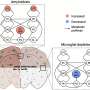
Research from UT Health San Antonio and the University of California at Irvine reveals that targeting brain lipid imbalances may offer new insights into Alzheimer’s disease treatment. The study highlights the significant role of lipid changes in the brain’s immune cells and how these alterations influence the disease’s development and progression.
The foundational work of Alois Alzheimer more than a century ago identified unusual brain fat changes alongside amyloid-beta plaques and tau protein tangles. While much research has focused on these proteins, the new findings emphasize that lipid abnormalities warrant closer attention. According to Juan Pablo Palavicini, Ph.D., an assistant professor at UT San Antonio’s Long School of Medicine, the brain is distinct in its composition, with over half of its dry weight consisting of lipids such as cholesterol and phospholipids. He stated, “In Alzheimer’s disease, we see massive disruption of these lipids, yet most studies focus only on genes and proteins.”
The findings, published in Nature Communications, demonstrate that microglia, the brain’s immune cells, play a crucial role in regulating lipid balance. Depending on their manipulation, these cells can either help to maintain lipid homeostasis or exacerbate the disease.
Investigating Microglial Impact
Utilizing a mouse model of Alzheimer’s disease, the research team tested two methods to eliminate microglia. One approach involved administering a drug that nearly eradicated all microglia, while the other utilized genetically modified mice lacking these cells. This setup allowed the researchers to differentiate the effects of microglia from those of other brain cells.
Palavicini noted, “We wanted to understand which cells are driving these lipid changes.” Their investigations revealed that amyloid accumulation significantly altered brain lipid profiles, particularly affecting two lipid groups: lysophospholipids (LPC and LPE), associated with inflammation, and bis(monoacylglycero)phosphate (BMP), which is vital for cellular recycling processes. The study discovered that a specific BMP variant, containing arachidonic acid (AA-BMP), accumulated near amyloid plaques. This accumulation was prevented by long-term microglial removal, suggesting that microglia are key drivers of these lipid changes.
The Role of Progranulin
Another important finding of the study was the protein progranulin, produced by both microglia and neurons, which emerged as a critical regulator of lipid metabolism. Researchers found that progranulin levels increased in Alzheimer’s conditions and correlated closely with AA-BMP levels. The removal of microglia resulted in decreased levels of both progranulin and AA-BMP near amyloid plaques, indicating that microglial-derived progranulin is essential for maintaining lipid balance.
Palavicini remarked, “In the Alzheimer’s brain, rather than lowering BMP, it may be important to maintain or support its levels.” The potential therapeutic implications are significant: boosting progranulin levels could help restore lipid balance and support neuronal health.
Notably, the research also highlighted that not all lipid changes are dictated by microglia. The levels of LPC and LPE were primarily influenced by astrocytes and neurons. LPC accumulation was linked to astrocyte activation, while LPE increases were associated with oxidative stress. “This distinction helps us understand which cells to target for therapies and shows how complex lipid regulation is in Alzheimer’s disease,” said Palavicini.
The study also revealed that microglia play a protective role for myelin, the protective sheath around neurons. Genetic removal of microglia under amyloid stress led to a reduction in myelin-related lipids, suggesting that these immune cells are essential for neuronal protection.
This research underscores that Alzheimer’s disease involves more than just amyloid plaques and tau tangles. It also includes disrupted lipid balance, with microglia, astrocytes, and neurons each contributing uniquely to the disease process.
Palavicini concluded, “Understanding which cells regulate which lipids opens the door to more precise therapies. By targeting lipid balance along with amyloid and tau, we can develop better strategies to protect neurons and potentially slow or prevent Alzheimer’s disease.”
The implications of this study are profound, paving the way for innovative approaches to Alzheimer’s treatment that focus on restoring lipid balance in addition to addressing the more traditionally studied protein-related mechanisms.