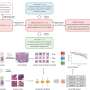
Researchers from UCL and UCLH have demonstrated that artificial intelligence (AI) can enhance the prediction of treatment outcomes for patients with rectal cancer. Their study, published in eBioMedicine, reveals that AI can analyze standard tissue samples taken during diagnosis to assess how well patients are likely to respond to therapies.
The immune landscape surrounding a tumor is crucial in understanding cancer progression and treatment responses. Yet, the intricate interactions between immune cells, tumor cells, and therapies often remain elusive. This study leveraged AI to analyze routine pathology images, measuring the types and abundance of key immune cells within the tumor microenvironment. This innovative approach aims to predict patient survival and the likelihood of disease recurrence.
The researchers focused on pathology slides, which are routinely examined by pathologists under microscopes. They sought to determine whether AI could effectively identify immune cell “signatures” in these images and correlate them with patient outcomes, significantly reducing analysis time.
Dr. Charles-Antoine Collins-Fekete, a senior author of the study from UCL Medical Physics & Biomedical Engineering, remarked, “Pathology slides are already part of routine care, so they’re an abundant source of data. We predicted that we could extract valuable information about a patient’s tumor from these slides using AI, which has become very good at analyzing medical images in recent years, and link this to patient outcomes.”
The findings indicate that AI can detect essential immune signals from pathology slides within minutes, compared to traditional methods that can take days and are often more costly, such as whole-genome sequencing or spatial transcriptomics. This capability could pave the way for more personalized diagnostic and treatment protocols, ultimately improving patient outcomes.
The study analyzed samples from three patient groups, including participants in the ARISTOTLE clinical trial. Results showed that patients with a higher presence of lymphocytes—immune cells that combat infections and diseases, including cancer—tended to enjoy longer survival and lower rates of cancer recurrence. Conversely, an increased presence of macrophages, another type of immune cell, correlated with poorer outcomes, as these cells can inadvertently support tumor growth.
These immune characteristics are not commonly factored into clinical decision-making for rectal cancer but hold the potential to guide personalized chemoradiotherapy and identify patients at greater risk for recurrence.
To assess the effectiveness of the AI system, researchers trained it using millions of pathology images and subsequently tested it on 900 patient samples. The AI could accurately measure immune cell levels before and after treatment. Patients exhibiting an increase in tumor-infiltrating lymphocytes—indicating a robust anti-tumor immune response—generally had better outcomes. In contrast, those whose tumors remained “cold” immunologically after therapy faced a higher likelihood of earlier recurrence.
The study also examined how genetic mutations in cancer impacted immune responses. Notably, patients with a normal KRAS gene and elevated lymphocyte levels had improved survival rates compared to those with KRAS mutations and fewer lymphocytes. Similarly, high macrophage levels proved particularly detrimental in patients with mutations in the TP53 gene.
Dr. Zhuoyan Shen, the study’s first author, highlighted the importance of integrating immune features into treatment decisions. “While experienced pathologists can recognize some immune features of the tumor microenvironment, this information is not routinely used to inform treatment. The AI approach identifies these hidden immune ‘signatures’ directly, offering a level of biological insight normally only attainable through methods like whole-genome sequencing.”
The researchers also noted that tumors characterized by high mitotic activity—indicating rapid cell division—tended to suppress immune function, leading to worse patient outcomes. This suggests that aggressive cancers may pose greater challenges for the immune system to combat.
To facilitate the use of their findings in clinical practice, the team has developed a free online tool called Octopath. This platform allows clinicians to upload pathology slides and receive automated immune analyses. Nevertheless, the researchers emphasized the need for further studies involving larger and more diverse patient populations to validate their results. They plan to investigate additional immune cell types and utilize advanced techniques to deepen the understanding of cancer-immune interactions.
Professor Maria Hawkins, another senior author of the study and a consultant clinical oncologist at UCLH, expressed optimism about the potential of AI in cancer classification. “This is an early step towards the use of AI to aid the further classification of cancer, but it is promising and very exciting for clinicians like me to start to understand what it may lead to in the future. In future, clinicians and patients will discuss personalization of treatment using timely information provided by AI. However, further research is required to understand how best to integrate these biomarkers into everyday clinical practice.”
The research presents a significant advancement in the intersection of AI and oncology, potentially transforming how rectal cancer is diagnosed and treated, and ultimately improving patient care.