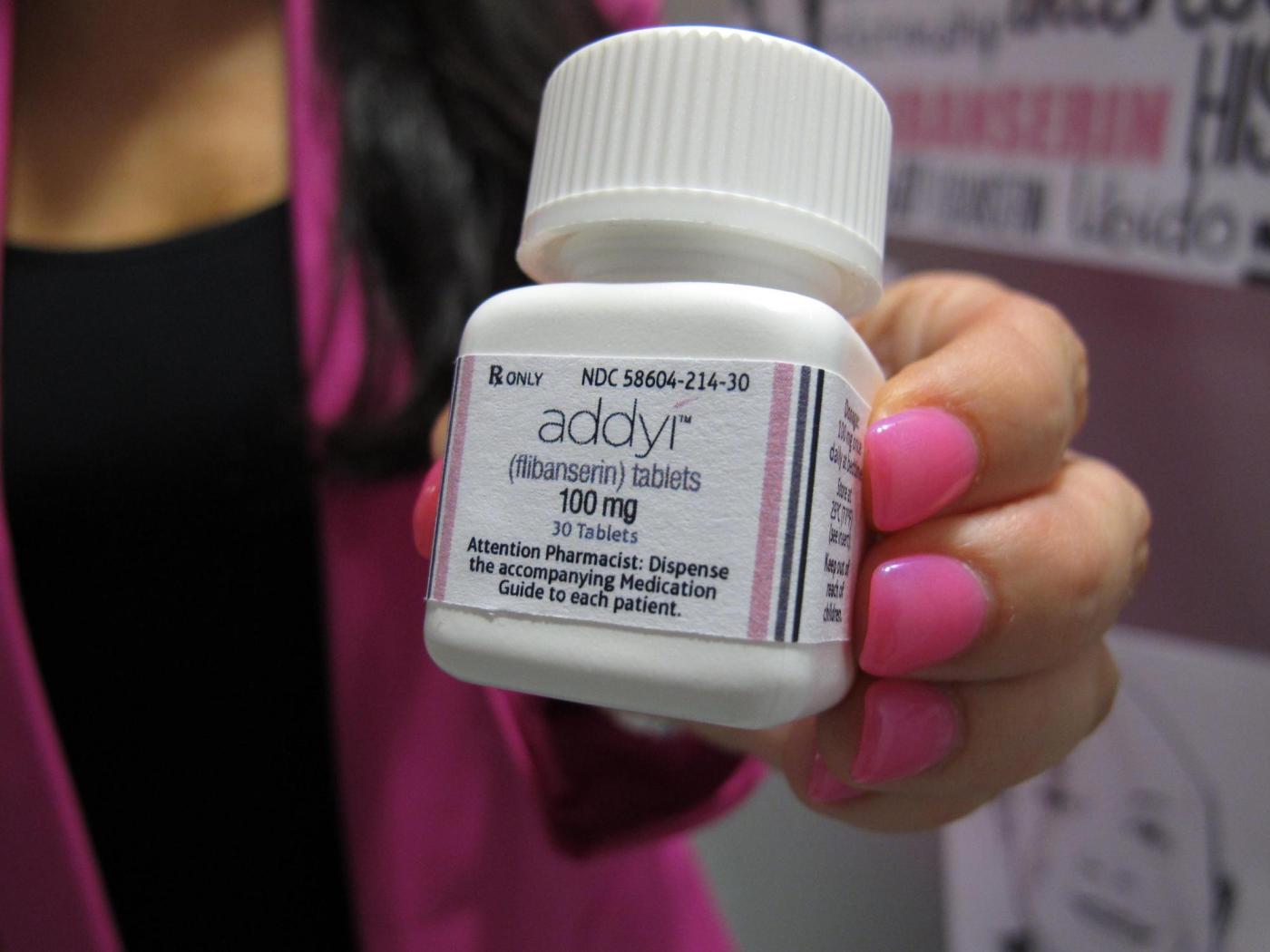
U.S. health officials have expanded the approval of the libido-boosting drug, Addyi, allowing its use in women over the age of 65 who have gone through menopause. This decision, announced on March 25, 2024, by the Food and Drug Administration (FDA), marks a significant change in the drug’s accessibility for older women experiencing low sexual desire.
Originally approved in 2015 for premenopausal women, Addyi was intended to address the challenges associated with hypoactive sexual desire disorder, a condition affecting a considerable number of women. Despite initial expectations of becoming a leading option in women’s health, Addyi faced criticism due to its side effects, which include dizziness and nausea. Notably, it carries a boxed warning cautioning against alcohol consumption while taking the medication, as this combination can lead to dangerously low blood pressure and fainting.
Sprout Pharmaceuticals, the manufacturer of Addyi, noted that the latest approval reflects ongoing efforts to change how women’s sexual health is prioritized. In a statement, Cindy Eckert, the company’s CEO, emphasized the importance of this decision, stating it represents a decade of collaboration with the FDA.
The medical community recognizes hypoactive sexual desire disorder as a legitimate condition since the 1990s. Surveys suggest that it affects a substantial portion of American women, prompting increased interest from pharmaceutical companies in developing treatments for female sexual dysfunction. Following the success of Viagra for men, many researchers began exploring therapies for women, but diagnosing this condition remains complex. Factors influencing libido can vary widely, particularly after menopause, due to hormonal changes and other medical symptoms.
Prior to prescribing medication such as Addyi, healthcare providers are required to rule out various factors that could contribute to low sexual desire, including underlying medical conditions, relationship issues, and mental health disorders. The diagnosis itself has sparked debate, with some psychologists questioning whether low libido should be classified as a medical issue.
The FDA previously rejected Addyi twice before its eventual approval, citing concerns over its modest effectiveness and potential side effects. This approval followed a vigorous lobbying campaign by Sprout Pharmaceuticals and its supporters, who framed the lack of treatment options for female libido as a matter of women’s rights.
Since its launch, sales of Addyi have remained limited. In 2019, the FDA approved a second drug for low female libido, an on-demand injection that operates through a different neurological mechanism. As the conversation around women’s sexual health continues to evolve, the recent FDA decision may pave the way for greater awareness and more treatment options in this often-overlooked area of healthcare.
The Associated Press Health and Science Department receives support from the Howard Hughes Medical Institute’s Department of Science Education and the Robert Wood Johnson Foundation, with all content produced independently by the AP.