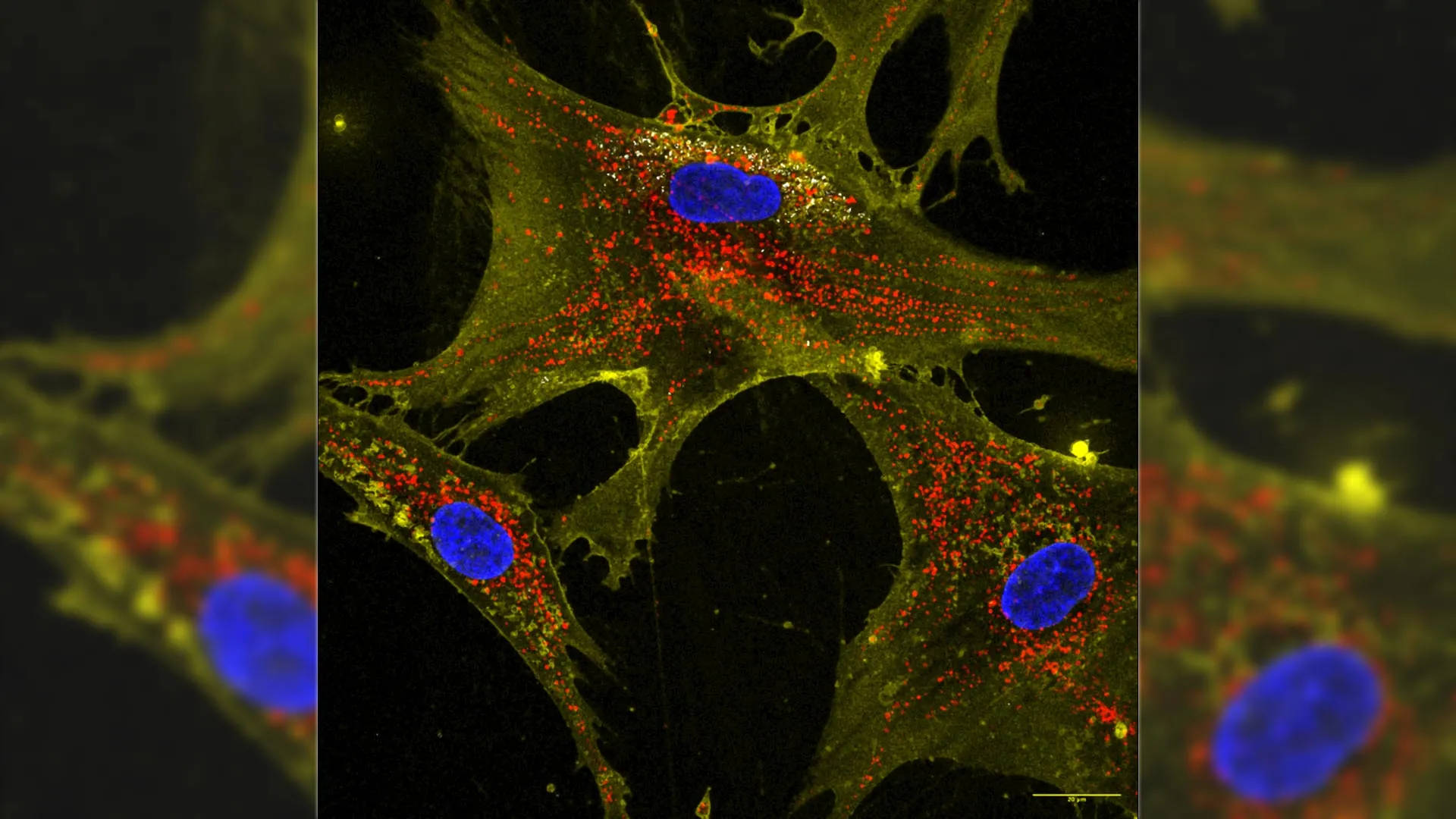
Researchers at Texas A&M University have developed a groundbreaking technique that enhances stem cells’ ability to produce mitochondria, potentially reversing the decline in cellular energy associated with aging and various diseases. This innovative use of microscopic particles known as nanoflowers enables stem cells to double their mitochondrial output and transfer these energy-producing organelles to damaged cells, leading to improved function and resilience.
The study, published on November 27, 2025, in the Proceedings of the National Academy of Sciences, marks a significant advancement in mitochondrial therapy. According to lead researcher Dr. Akhilesh K. Gaharwar and Ph.D. student John Soukar, the method could address energy deficiencies in a wide range of conditions, including aging, heart disease, and neurodegenerative disorders.
Nanoflowers Transform Stem Cells into Mitochondrial Donors
The research team successfully combined stem cells with flower-shaped nanoparticles, resulting in a marked increase in mitochondrial production. When these enhanced stem cells were placed next to aging or injured cells, they transferred the excess mitochondria, revitalizing the energy levels of their neighbors. The treated cells not only exhibited higher energy output but also demonstrated greater resistance to cell death, even after exposure to harmful treatments like chemotherapy.
Gaharwar explained the process, stating, “We have trained healthy cells to share their spare batteries with weaker ones.” This approach does not involve genetic modification or drug intervention, making it a promising alternative for cellular rejuvenation. Soukar elaborated on the efficiency of this technique, noting that the number of mitochondria transferred was two to four times greater than what untreated stem cells could provide. “It’s like giving an old electronic a new battery pack,” he added.
Potential for Long-Lasting Therapies
Traditional methods for increasing mitochondrial numbers often require frequent treatments, as they rely on drugs that quickly exit the cells. In contrast, the nanoflower technology utilizes larger nanoparticles, roughly 100 nanometers in diameter, which remain within the cells for extended periods. This could allow therapies to be administered less frequently, potentially once a month, while still maintaining effectiveness.
Gaharwar stated, “This is an early but exciting step toward recharging aging tissues using their own biological machinery.” If further studies validate these findings, the technique could fundamentally change how medical practitioners approach aging and degenerative diseases.
The nanoflowers are composed of molybdenum disulfide, a compound that can adopt various two-dimensional shapes at nanoscale. This research places Texas A&M at the forefront of exploring new biomedical applications for such materials, particularly in enhancing stem cell efficacy for tissue repair and regeneration.
The flexibility of this approach is one of its most promising features. Although the method is still in its infancy, Gaharwar and Soukar believe it could be applied to various tissues throughout the body. For instance, stem cells could be injected directly into the heart for cardiac issues or into muscles to address conditions like muscular dystrophy.
Support for this project came from a range of esteemed institutions, including the National Institutes of Health, the Welch Foundation, the Department of Defense, and the Cancer Prevention and Research Institute of Texas. Additional funding was provided by the President’s Excellence Fund at Texas A&M University and the Texas A&M Health Science Center Seedling Grant, with crucial contributions from fellow researchers Dr. Irtisha Singh, Dr. Vishal Gohil, and Dr. Feng Zhao.
The ongoing research into nanoflowers represents not only a significant step in cellular health but also a potential pathway to new therapeutic strategies for a variety of diseases. As the team continues their investigations, the possibility of using this technology to combat the effects of aging and restore cellular function remains a captivating prospect for the future of medicine.