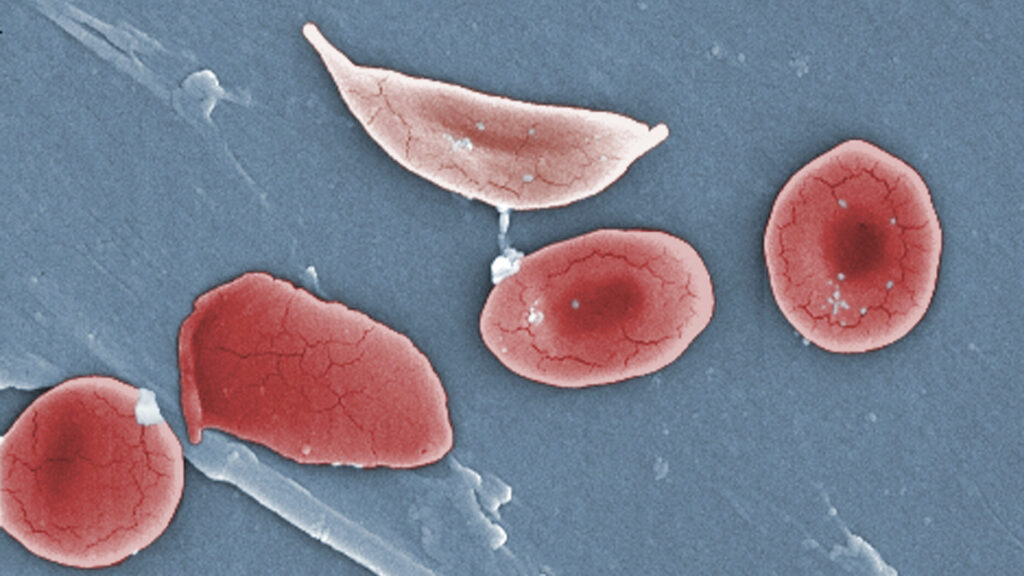
Vertex Pharmaceuticals is encountering significant delays in the rollout of its gene-editing therapy, Casgevy, for treating sickle cell disease. Despite receiving approval over two years ago, only around 60 patients across the U.S., Middle East, and Europe have been treated with this promising therapy. The company had previously indicated that the treatment’s availability would be gradual, but the current pace has surprised many in the medical community.
One of the primary challenges hindering the faster deployment of Casgevy is the difficulty in collecting sufficient cells required to develop the treatment. Specialists at several sickle cell centers reported that this issue has emerged as a major obstacle, ultimately slowing the process of providing the therapy to patients in need.
Vertex is navigating a critical juncture as it seeks to establish Casgevy in the market. The company now faces rising competition from alternative therapies and is preparing for a significant competitor anticipated to enter the market next year. This context adds urgency to the need for a more efficient patient treatment process.
The challenges surrounding cell collection are particularly concerning given the potential of Casgevy to change the lives of those suffering from sickle cell disease. Sickle cell disease affects millions globally, leading to severe pain and complications. The promise of a curative treatment offers hope to patients and families who have long awaited advancements in this area.
Vertex’s leadership remains focused on addressing these hurdles. The company is exploring solutions to optimize cell collection and improve the overall treatment timeline. The expectation is that, with time, the processes can be refined, allowing for broader patient access to this innovative therapy.
As Vertex continues to tackle these issues, the healthcare community watches closely, eager for developments that could enhance treatment options for sickle cell disease. The outcome of these efforts will not only impact Vertex’s standing in the competitive landscape but also the lives of patients who are in desperate need of effective therapies.