Research has uncovered the mechanism behind the rapid prey-capturing abilities of the Venus flytrap (Dionaea muscipula), a carnivorous plant known for its unique spring-loaded traps. According to a study published in Nature Communications by a team led by Hiraku Suda, this mechanism involves a specific mechanosensor known as DmMSL10.
The Venus flytrap, alongside other carnivorous plants like the waterwheel plant (Aldrovanda vesiculosa) and sundews, employs specialized sensory hairs that respond to tactile stimuli. These hairs use calcium threshold signals to trigger the trap’s closure. While the general function of these hairs was previously understood, the specific mechanisms remained largely elusive until now.
Understanding DmMSL10’s Role
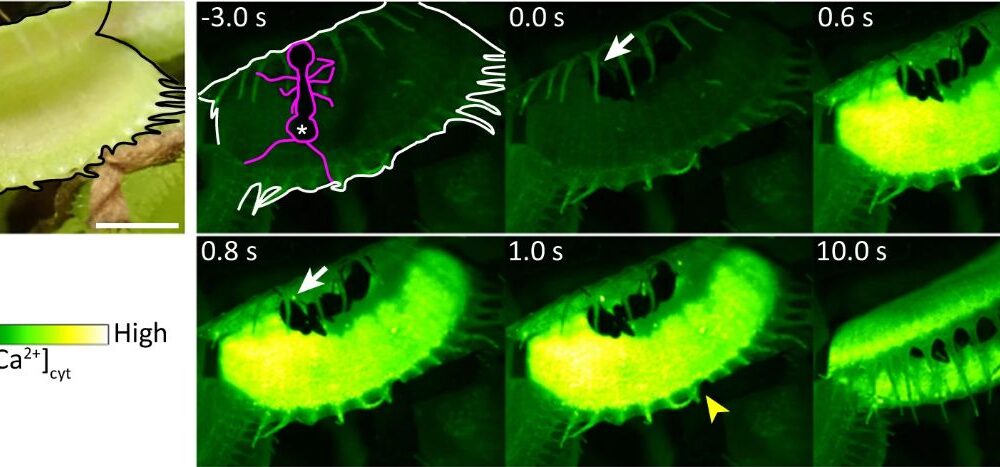
The research team created a variant of the Venus flytrap that lacked the DmMSL10 mechanosensor, which is a stretch-activated chloride ion (Cl–) channel. This absence drastically affected the plant’s responsiveness. Both the wild-type and knockout variants of DmMSL10 initiated calcium ion release when stimulated, yet the knockout variant showed a significantly lower rate of action potential generation.
In practical terms, this means that while the wild-type Venus flytrap continued to register action potentials even after stimulation stopped, the knockout variant did not respond effectively. This finding highlights the essential role of DmMSL10 in detecting subtle movements on the plant’s surface, such as the presence of prey.
Further experiments involved placing ants on the leaves of both the wild-type and the knockout plants. The wild-type trap successfully closed around the first ant, demonstrating its quick response to tactile cues. In contrast, the knockout variant failed to react, even after multiple ants attempted to wander across its surface, illustrating a clear deficiency in the calcium signaling required for prey capture.
Implications for Carnivorous Plant Biology
This new understanding of the DmMSL10 mechanosensor provides significant insight into how Dionaea muscipula and similar species manage to generate long-range calcium signals. These signals are crucial for the effective capture of prey, which consists primarily of protein and nitrogen-rich insects.
As researchers continue to explore these mechanisms, the findings may shed light on the evolutionary parallels between plant and animal response systems. The study not only enhances the scientific community’s understanding of plant biology but also opens avenues for further research into the evolutionary adaptations that enable such sophisticated predatory behaviors in plants.
With this discovery, scientists can now better appreciate the intricate interplay of biology and evolution that has allowed carnivorous plants to thrive in environments where nutrients are scarce. The implications of this research may extend beyond botany, potentially informing studies in other fields, including ecology and evolutionary biology.