Researchers at Karolinska Institutet have unveiled new insights into the protein p53, often referred to as the “guardian of the genome.” This study reveals that p53 can influence blood vessel growth in contradictory ways, acting to both slow and, at times, damage these vessels. The findings, published in the journal Cell Death & Disease, highlight the complexity of p53’s role in vascular biology.
Revealing p53’s Dual Effects on Blood Vessels
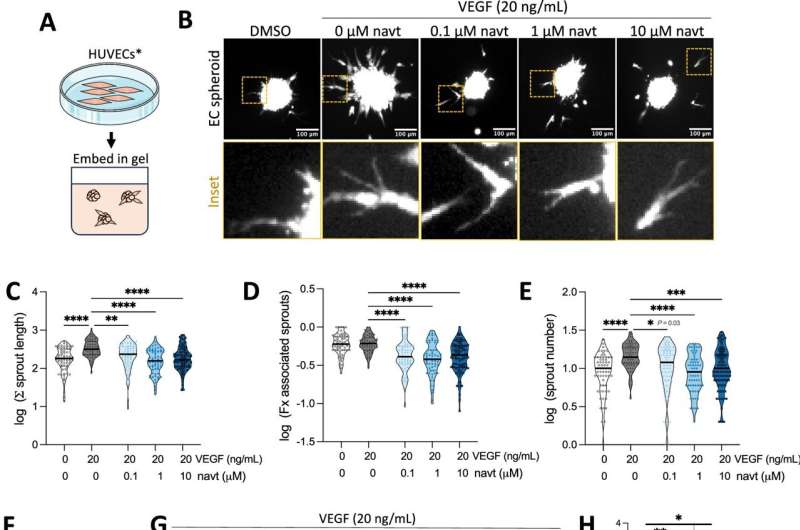
Utilizing advanced imaging techniques and a novel class of compounds, the research team investigated how varying levels of p53 affect the behavior of healthy blood vessel cells during the formation of new vessels. The researchers observed that p53’s response strength is critical in determining its effects, which can lead to drastically different cellular outcomes.
During the study, the team noted that specialized cells in the blood vessel walls engage in a coordinated process of division and movement to establish new vessels. However, in conditions such as cancer and certain eye diseases, this process can become uncontrolled, exacerbating these medical issues.
According to Pavitra Kannan, a researcher within the Department of Microbiology, Tumor, and Cell Biology at Karolinska Institutet, “One of the most striking observations was how sensitive these blood vessel cells are even to very low p53 levels compared to other cell types.” This sensitivity underscores the importance of p53 in regulating cell division.
Implications for Future Research
The study findings indicate that lower levels of p53 can temporarily halt the division of blood vessel cells. In contrast, elevated p53 levels can lead to permanent states where these cells are unable to divide or survive. Despite these contrasting responses, both low and high levels of p53 were found to reduce blood vessel growth.
This research enhances the understanding of p53’s diverse roles in blood vessel dynamics, showcasing how a single protein can produce vastly different cellular outcomes based on its activation level. The implications of these findings are significant, particularly for future therapeutic strategies aimed at controlling abnormal blood vessel growth associated with cancer and specific eye diseases.
The full details of this study can be found in the article by Omayma Al-Radi and colleagues, titled “Pharmacological activation of p53 induces dose-dependent changes in endothelial cell fate during angiogenic sprouting,” published in Cell Death & Disease on January 2, 2026. The research contributes to ongoing efforts to better understand vascular biology and its implications for health and disease.