Researchers at Rice University have developed a groundbreaking method to control seizure-related brain activity without the need for surgical intervention. This innovative approach, which utilises sound waves and gene therapy, targets specific brain circuits linked to epilepsy, offering a potential new avenue for treatment. The study, recently published in the journal ACS Chemical Neuroscience, demonstrates the efficacy of this technique in animal models.
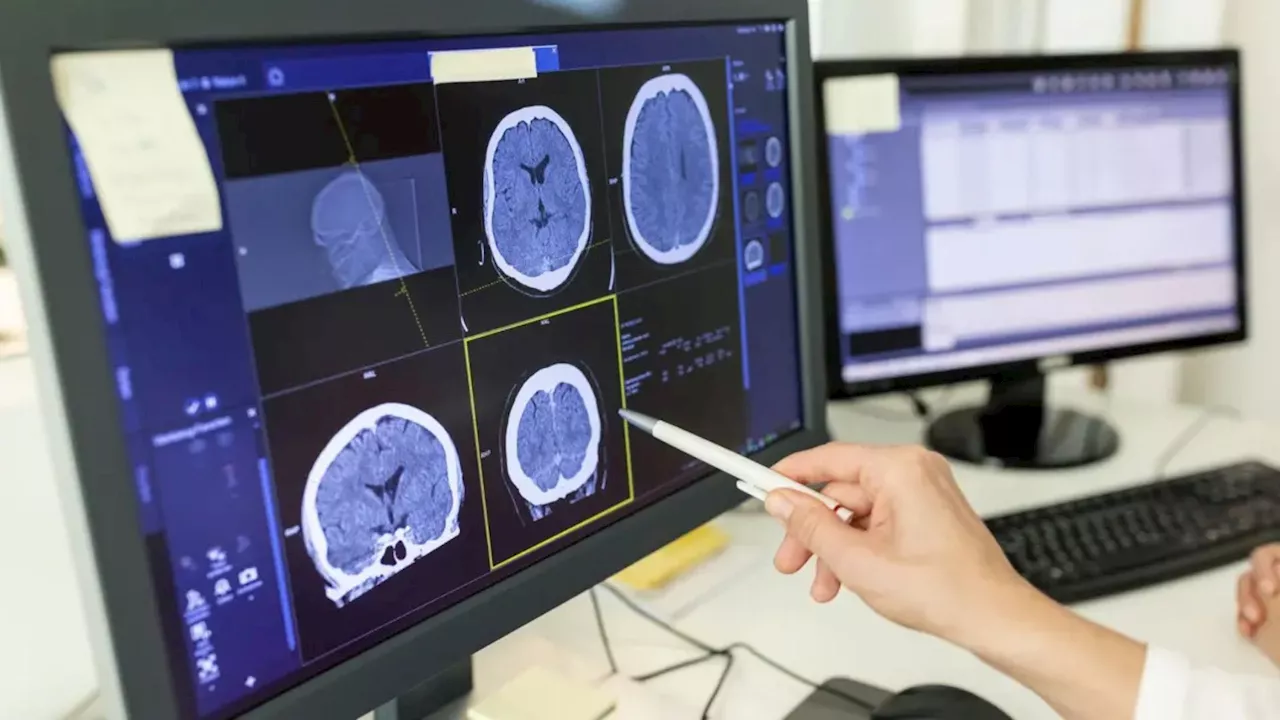
At the heart of the research is the use of low-intensity focused ultrasound to temporarily open the blood-brain barrier, allowing targeted delivery of gene therapy to the hippocampus. This brain region is often associated with seizure activity. The method, termed acoustically targeted chemogenetics, or ATAC, enables researchers to modulate neuronal activity without affecting surrounding areas.
Led by Jerzy Szablowski, an assistant professor of bioengineering and a member of the Rice Neuroengineering Initiative, the research team injected tiny, gas-filled bubbles into the bloodstream. When ultrasound waves were directed at the hippocampus, these bubbles created temporary openings in the blood-brain barrier, allowing gene therapy vectors to penetrate the targeted tissue.
“In many neurological diseases, hyperactive cells in specific brain locations drive the condition,” Szablowski stated. “Our technique targets the therapy precisely where it is needed and provides control without the complications of surgery or permanent implants.”
The engineered vectors carry genetic instructions for an inhibitory chemogenetic receptor, effectively acting as a “dimmer switch” for neurons. This allows scientists to selectively activate or deactivate specific neurons with an oral medication designed to dampen overactivity.
Honghao Li, a doctoral student in bioengineering and the study’s first author, explained the significance of this targeted approach. “By precisely targeting the hippocampus, we can reduce overactivity where it matters and leave the rest of the brain untouched,” he said.
The findings indicate that ATAC can effectively control brain circuits through a minimally invasive procedure and a simple drug regimen. Both the focused ultrasound and viral vector gene delivery components are currently undergoing clinical trials, suggesting that this method could expedite the development of new treatments for epilepsy and other neurological disorders.
The research marks a significant advancement for Szablowski’s team, which is also focused on further innovations in gene therapy delivery. They have developed another ultrasound-based method called recovery of markers through insonation, or REMIS, which facilitates the release of proteins from specific brain areas into the bloodstream for monitoring purposes.
“These technologies complement each other,” Szablowski noted. “Ultrasound enables us to deliver therapy, control targeted neurons, and measure effects in the specific circuits we aim to influence.”
As the research progresses, the ultimate goal is to create a versatile platform capable of safely reaching any brain region, delivering genetic material precisely, and allowing clinicians to control it as needed. This work underscores Rice University’s expanding focus on brain science and neurological health, now consolidated under the newly established Rice Brain Institute.
The potential implications of this research extend beyond epilepsy, offering hope for various neurological disorders where precise control of brain activity is crucial. The innovative combination of sound waves and gene therapy could revolutionize treatment options and improve the quality of life for those affected by such conditions.